What is an emulsifier?
An emulsifier is amphiphilic molecule which is composed of a lipophilic tail (affinity for oil) and a hydrophilic head (affinity for water). This characteristic makes it possible to reduce the surface tension between two immiscible liquids such as oil and water. The use of an emulsifier and shear strength generates an oil-in-water or a water-in-oil dispersion.
Consequently, when bitumen and oil are mixed in the presence of an emulsifier, shear strength of the colloid mill produces bitumen droplets into water: a bitumen emulsion with a stability controlled in time.
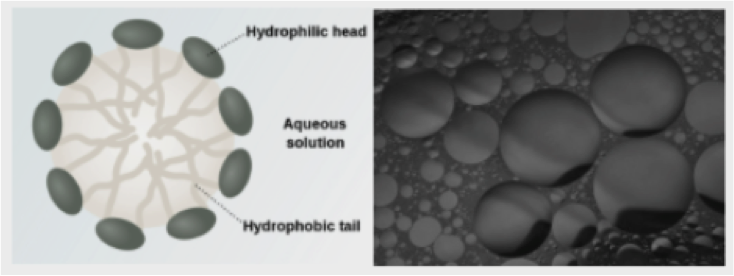
Since the continuous phase of a bituminous emulsion is water, its viscosity is significantly lower than bitumen. This enables the handling of bituminous materials at ambient conditions in opposition to hot or warm techniques.
There are 3 categories of bitumen emulsifiers:
- Cationic: their hydrophilic head is positively charged.
- Anionic: their hydrophilic head is negatively charged.
- Amphoteric: their hydrophilic head is both positively and negatively charged. This way, they can perform either like cationic emulsifiers or anionic emulsifiers depending on the pH.